Ongoing research indicates that chronic adrenal insufficiency patients are suffering higher than expected mortality, as well as other long-term side effects, such as obesity, difficulty loosing weight, glucose intolerance, diabetes, muscle wasting, cardiovascular disease and osteoporosis. This results primarily from the highly un-physiological glucocorticoid replacement therapies currently available. There has been limited treatment innovation for decades in this field, and there is a clear need for an improved regiment that more adequately mimics the diurnal profile of cortisol. Extreme interest in the long-term outcomes for patients with adrenal insufficiency led researchers at Gothenburg and Uppsala Universities in Sweden, to begin work on a novel replacement medication, DuoCort, that contains both rapid release and extended release portions that when combined in one tablet, can closely mimic normal cortisol secretion.
Introduction
The hormone cortisol has gained widespread attention as the so-called “stress hormone” because it is released in the body during stressed or agitated states. But this hormone is necessary for the functioning of almost every part of the body. Excesses or deficiencies of this crucial hormone can lead to various physical symptoms and diseases. The body possesses an elaborate feedback system for controlling cortisol secretion and regulating the amount of cortisol in the blood.
The body’s normal level of cortisol secretion occurs in a diurnal cycle. In other words, cortisol levels vary during the 24-hour day. The most active period occurs during the day, starting with the highest concentrations in the early morning, and tapers off to the lowest by about midnight. In addition to this normal rhythm, cortisol secretion increases in response to any stress, whether physical or psychological
Regardless of the cause of adrenal insufficiency (Addison’s disease, removal of the adrenal glands to cure Cushing’s, hypopituitarism or prolonged exposure to glucocorticoids) treatment of adrenal insufficiency involves replacing, or substituting, the hormones that the adrenal glands are not making. Cortisol is replaced with hydrocortisone, the synthetic form of cortisol. This is administered in tablets taken two or more times a day. During replacement therapy, both the total daily dose and the pattern of its delivery are important. Synthetic glucocorticoids have also been used for this purpose, but the dose titration is less reliable and risk of side-effects related to glucocorticoid excess is increased.
Conventional Replacement
Today’s conventional tablets are incapable of mimicking the body’s natural cortisol rhythm, with the result that patients experience both too low and too high plasma levels of cortisol as they go through the day. The total daily replacement dose is often divided into two or three doses throughout the day, for example, 15 mg at 8:00 a.m. and 10 mg at 3:00 p.m. Most of the daily dose is typically administered in the morning. The problem is that this first morning dose, being a conventional tablet, will require 1-2 hours to be absorbed from the gut and fully bio-available. The later dose or doses are prone to being forgotten or delayed. The result is overly high serum levels 2-4 hours after the ingestion of the tablets and low or undetectable levels in-between. This non-physiological pattern of cortisol replacement often requires total daily replacement doses that exceed the amounts actually needed for the healthy functioning of the body. With long-term treatment, these cortisol replacement levels can lead to obesity, osteoporosis cardiovascular disease, and other conditions associated with excess cortisol resembling the Cushing syndrome. The trough levels in between doses can increase the risk of adrenal crises in stress situations and can increase the risk of insufficient immune response to opportunistic infection. In some cases, the synthetic corticosteroids prednisolone and dexamethasone are used to replace cortisol, rather than hydrocortisone. However, these are much more potent steroids than hydrocortisone and the use of these drugs easily leads to overdosing. They are nevertheless used in preference to hydrocortisone in order to achieve once-daily dosing.
In summary, the main problems with present treatments are: 1) By virtue of the dosage forms, patterns of administration, and type of glucocorticoid used, the total daily dose of glucocorticoid replacement is too high in many patients, 2) Existing administration forms cannot adequately mimic the 24 hour circadian pattern of cortisol, 3) Multiple daily dosing can result in patient failure to fully comply with treatment, exposing the patient to risk and sometimes requiring extra dosing to correct.
Improved Replacement
With its dual-release mode of action, DuoCort™ will address several clinical problems. By achieving significant serum levels of cortisol in less than half the time of current therapy after morning administration followed by an extended release over the day, DuoCort™ therapy will closely mimic the natural circadian biological rhythm of cortisol (see below).
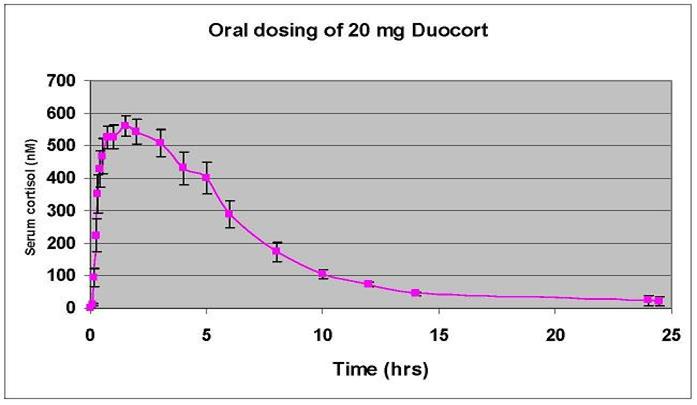
The benefit of a more physiological cortisol therapy is both better patient quality of life and better long-term outcomes in terms of the side-effects related to cortisol excess (obesity, glucose intolerance, diabetes mellitus, muscle wasting, and osteoporosis). With a more physiological cortisol replacement therapy the patient will not get the cortisol peak they currently experience 2-3 times a day with conventional therapy, although they will continue to get their morning peak but much more quickly with DuoCort™. Low or undetectable cortisol levels during the day are also avoided through the extended release of the DuoCort™ product, thereby minimizing fatigue and other risks. By enabling patients to take their medicine only once per day, rather than two or three times per day, the DuoCort™ product enhances convenience for patients and improves prescription compliance. Each of these aspects will be evaluated in the current clinical trial and in future clinical trials.
Current Status
DuoCort™ is currently in late-stage clinical trials in Europe and is expected to be launched there in 2010. DuoCort™ comes in two dosage strengths, 5 mg and 20 mg, which were chosen in order to address probably the most common total daily dose (20 mg) while giving the flexibility to address other daily doses by combining 5 mg and 20 mg tablets. Other dosage strengths are planned.
DuoCort™ has completed a Phase I clinical study and has obtained an orphan drug designation in the European Union. A Phase II/III clinical study is underway and based on discussions with the European Medicines Agency (EMEA) is expected to be the basis for European registration in 2009 as an orphan drug provided the end points of the study are met. A program for pediatric clinical trials is in preparation, as is a plan for availability in the U.S.
Enrolment of patients into the pivotal phase II/III clinical trial evaluating 5 mg and 20 mg DuoCort™ dual-release hydrocortisone tablets for the treatment of adrenal insufficiency was completed in December 2007. The first part of the study involves a comparison of DuoCort™ therapy to three-times daily therapy with conventional hydrocortisone tablets in a cross-over design in which patients spend 12 weeks on one therapy and then switch to spend 12 weeks on the other. There is no data from the trial yet, but anecdotal reports from the trial centers indicate that DuoCort™ is well-tolerated and all of the patients on DuoCort™ therapy are doing well. Patients using DuoCort™in situations of intercurrent illness and other stresses report no problems. After the cross-over part of the trial, the patients will enter a six-month open extension on DuoCort™, during which various secondary endpoints will be measured. The patients will be offered an open extension on DuoCort™ following the end of the trial.
Quality of life data, using four different patient questionnaires, is being collected throughout the full 12 months of the trial and will continue to be collected in the open extension arm. This is a very important aspect, among others, that will need to be followed in long-term studies after DuoCort is commercially available.
Conclusion
In Phase I clinical trials, DuoCort was shown to provide true, once daily oral hydrocortisone replacement that mimicked the natural 24-hour circadian rhythm, resulting in lower total cortisol exposure for patients and improved quality of life. Lower total cortisol exposure translates into fewer side effects related to cortisol excess and a once daily dosage improves patient compliance. In addition, the shorter time to reach clinically relevant concentrations can improve patients functioning early in the day. DuoCort has been shown to be ideal for dose supplementation in case of illness, stress and similar events.
Authors: Dr. Gudmundur Johannsson, MD, PhD and Greg Batcheller (Spring 2008)
Editor’s Note: Dr. Gudmundur Johannsson, MD, PhD, is Consultant and Head of Department of Endocrinology, Sahlgrenska University Hospital, and Senior Lecturer at Gothenburg University, Sweden. Dr. Johannsson’s research interest in long-term outcomes for patients with adrenal insufficiency led him, along with other scientists, to found DuoCort AB, a Swedish endocrinology company to develop an improved therapy. Greg Bactheller is CEO of DuoCort AB. DuoCort has been acquired by ViroPharma. DuoCort is being marketed in Europe (2012) under the brand name Plenadren. More information can be obtained through the company’s website at www.DuoCort.com.




Sorry, comments are closed for this post.