By: Leslie Edwin and Amy Dahm
Two delegates – Leslie Edwin, President, and Amy Dahm, “DC Correspondent” – represented the CSRF at the National Organization for Rare Disorders (NORD) Orphan Products Breakthrough Summit at the Marriott Wardman Park in Washington, DC October 14-16, 2018. Sam, Amy’s service dog, accompanied them to the conference and information sessions. The NORD Summit is one of the most important meetings of the year because it is the only platform that brings scientists, researchers, patient advocacy organizations, finance, pharma, government, regulators, and clinicians all under one roof. It’s a great time to learn about best practices, legislation, and innovations that affect hundreds of thousands of people in the rare disease community.
NORD Members Pre-Conference Meeting
The day before the official launch of the conference, we attended the NORD members-only Pre-Conference Meeting. NORD representatives staffed themed networking tables, and delegates discussed topics such as Patient Assistance Programs in depth. One of the highlights was a panel featuring case studies of different organizations’ social media fundraising strategies using FaceBook, Twitter and YouTube. Panelist Vanessa Vogel-Farley, Executive Director of rare disease patient group Dup15q Alliance, emphasized the importance of building a strong donor community by utilizing social technology. Small rare disease groups have common challenges when it comes to fundraising, and these pre-conference meetings are excellent opportunities to network and see what others are doing to fund their efforts to improve outcomes for patients.
Relatedly, another panel, “Collaboration with Industry: Partnerships, Potential, and Pitfalls,” examined the benefits and challenges of patient organizations partnering with industry. This topic frequently finds itself in critics’ crosshairs, but the scrutiny is an opportunity to achieve greater transparency. Partnerships with companies who make specialty drugs for tiny populations such as ours can be mutually beneficial. There are thousands of rare diseases that have zero drugs created solely to treat their condition, and manufacturers of generic medications and those that are used off-label do not seem to make the same investment in educating and supporting their rare communities.
Main Conference Opening Remarks and Young Advocates

Peter Saltonstall, President and CEO of NORD, opened the conference with a brief welcome and summit preview. The theme for 2018 was “A New Era of Patient-Focused Innovation”. There does indeed seem to be a ramping up of interest in patient-focused outcomes and the patient voice influencing research, so we were excited to see NORD’s focus on the topic. This welcome implored all stakeholder attendees to invest in the human relationship in medicine and to take time to get to know patients.
Every year immediately following Mr. Saltonstall’s opening address there is a Young Advocate panel that showcases the next generation of patient advocacy and discusses ways patients are assuming innovative roles to help advance research. Four young adults – Gabriel Low, Taylor Kane, Harjot Randhawa, and Christopher Anselmo – shared their personal stories of how they became involved in rare disease advocacy. Scan the QR code to learn more about each (web addresses for more information about these three are included in the footnotes of this article if you do not use a QR reader). Dr. Anita Gupta, a physician and rare disease survivor, moderated the panel and stated that “somewhere in all the education, we sometimes forget empathy and compassion. Sometimes it’s becoming a patient that makes us appreciate the experience of a patient.”
How Patients are Helping Drive Research and Drug Development
There are many ways that patient input and involvement in research will improve outcomes for current and future patients. Simply lending your voice to a survey or questionnaire could have an enormous impact – the information is used by researchers who ultimately publish the studies that are used by clinicians to, for example, figure out what to do when they encounter the first Cushing’s patient of their career.
Patients bring focus, relevance, and urgency to the research process. Scientists are curious, but when working with patents they can be more likely to adhere to the single problem they are addressing in the trial; they may not be aware of other problems to address. To get a seat at the table, we have to establish the fact that we are the authorities on our disease. We need to attend meetings and reach out. It is vital to make sure at all times that clinicians, researchers, and pharma are clear on what we, the patient population, actually consider the most important outcomes. Collaboration is essential to everyone’s ability to work at maximum effectiveness.
There are about 250 new rare diseases defined every year, and there are currently about 7000 known rare diseases. There is no sign that these numbers will decrease; genetic research is increasing this number more than anything. These large numbers can be daunting when taken as a whole, so it is recommended to “act locally, think globally”. Many good ideas for single patient communities can be adapted for others.
Solving the Diagnosis Challenge
Shortening delays in diagnosis is a huge challenge facing not only Cushing’s patients but the rare disease community as a whole. Big opportunities for genetic research, especially over the last decade, have come from efforts to address unmet needs and other delays in diagnosing a rare disease. The National Institutes of Health (NIH) has an Undiagnosed Diseases Program (UDP), which is part of a larger national network aimed at finding and diagnosing patients who have been passed around multiple specialists with no conclusive testing or discovery. The NIH reports that the majority of successful diagnoses through this program are the result of physicians coming together to discuss cases and crowdsource their wisdom and experience, not from testing alone. You can learn more about the UDP by scanning the QR code or visiting https://www.genome.gov/27543155/udp-background/.
Using Technology and Artificial Intelligence (AI): There’s an App for That
One of the most fascinating sessions explored how to use technology and AI to assist doctors in diagnosing and identifying rare disease and to help patients shorten their diagnostic timeframe. Dr. Christine Stanley, Head of the US Clinical Laboratory for WuXiNextCode, presented on how to integrate phenotype to diagnose more patients (yourdictionary.com explains the difference between genotype and phenotype well – genotype is the set of genes in our DNA which is responsible for a particular trait, while phenotype is the physical expression, or characteristics, of that trait). Dr. Marius Lingararu from Children’s National Health System discussed the development of an app that can scan the features of a newborn’s face and determine whether the infant is at low, medium, or high risk of having a particular disease. This early detection of dysmorphology (birth defects) helps to accelerate diagnosis so patients have access to treatment at the earliest possible opportunity.
Oodaye Shukla, Chief Data Scientist at analytics company HVH Precision, discussed an emerging technology that allows its scientists to analyze large data sets to find patient pools that have symptoms consistent with that disease to ultimately lead to quicker diagnoses, especially for rare diseases. HVH is looking to partner with Cushing’s patients who could help inform and guide a patient-based collaboration. If you are interested, please scan the QR code or visit HVH’s website at https://www.hvhprecision.com for more information about the company, then contact Amy Dahm at [email protected].
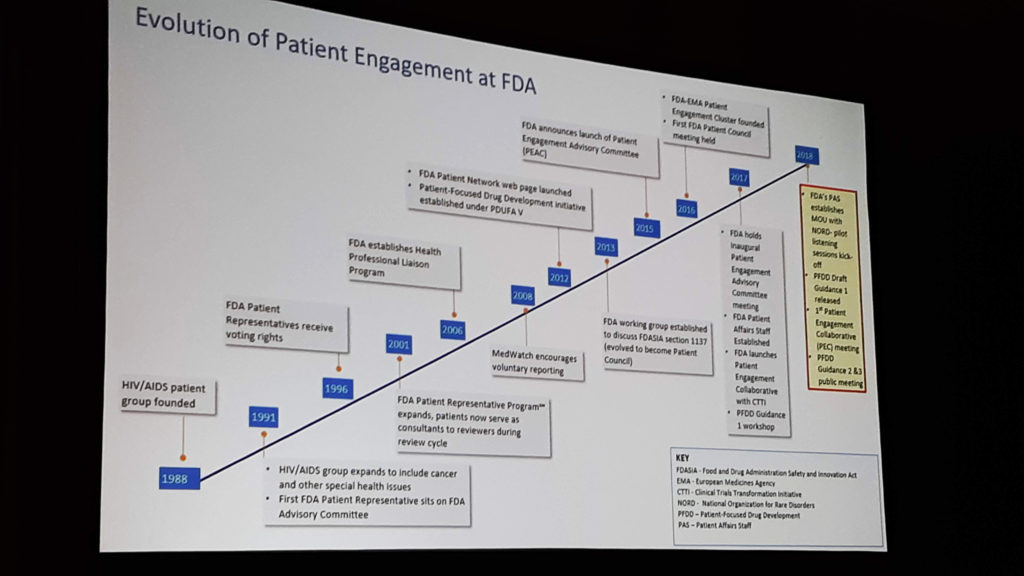
Food and Drug Administration (FDA) Commissioner Keynote
Dr. Scott Gottlieb, FDA Commissioner (has since resigned the position), was scheduled to give the keynote address but was called away the morning of the meeting for an emergency elsewhere in DC. Dr. Rachel Sherman, Principal Deputy Commissioner of the FDA, stepped in and delivered the keynote on the topic of “NORD Membership: Integrity, Responsibility, Strength.”
Dr. Sherman talked about another topic that is getting a lot of attention right now – patient registries. These databases of patient-reported information are extremely important and as such are one of very few areas in which the FDA gives grants. If you want a few excellent examples of the type of research that can be pulled from registries, please scan the QR code or search PubMed (https://www.ncbi.nlm.nih.gov/pubmed) for Dr. Elena Valassi’s work using the European Register on Cushing’s Syndrome – (ERCUSYN).
The mission of the FDA is to get products to patients, and they are constantly expanding ways for patients to connect and participate in the process. To challenge the idea that patient participation in FDA processes or genetic testing is out of reach for most, she pointed out that over 15 million consumers have purchased DNA kits from online retailers like 23andMe and Ancestry. If it was possible for actual genetic counselors to access this information, who knows what significant breakthroughs could be achieved. It might be a bit of oversimplification, but the point is correct that patient participation in genetic mapping could be “just as easy”.
The Patient Fair
Every year there is an open fair showcasing resources, drugs, and organizations that assist patients. One of the organizations, Patient Airlift Services (PALS), based in Farmingdale, NY, offers free air transportation to patients needing specialized treatment or follow-up at a distant facility, patients who have a large number of visits, and those in fragile health who must avoid public places because of compromised immune systems. PALS’ number is 631-694-7257 and website is http://www.palservices.org/, or scan the code.
Federal Policy Priorities and Rare Disease Legislation
Unfortunately, the picture is not very clear right now out of Washington. NORD’s position is that the issue of drug costs is so massive that not much is likely to change any time soon. Matters of adequate and affordable insurance aren’t going anywhere, either. Data suggests that the significant rate increases seen in 2018 were “too high and based on actuarial-predicted inflated costs that did not end up happening”. Since another major election looms now, it is likely that a lot of chatter will swell on this topic because it is a hot button for candidates to discuss their stances.
It is confusing to try to decipher biased summaries about legislation up for discussion and vote; a good example of this would be the Right to Try law (Senate Bill 204) that was signed into law in May 2018. This law allows patients to access investigational drugs outside of clinical trials if there are no other treatments available and they can’t participate in a trial. Usually these patients are extremely sick. NORD was very vocally opposed to this bill, which might sound odd at first considering they advocate for rare patient access to medications. The opposition came from a stance, supported almost unanimously by member organizations, that an already vulnerable group of people (very sick, rare) would become more exposed to dishonest dealings. The FDA already approves 99.5% of applications for emergency drug needs through their Expanded Access program, sometimes on the same day they receive the application. So technically, they already have something in place for fast-tracking medicine to those in dire need of it.
If you are interested in participating in the legislative process, you do not need a lot of experience or special contacts to get involved with your state and district delegations. Even a single conversation can lead to building bipartisan support for rare disease issues. NORD’s Rare Access Networks now operate in all states, are volunteer-led, and are always looking for passionate individuals to help participate in, organize, and present rare issues locally. If this interests you, please scan the QR code or visit http://rareaction.org/get-involved/join-rare-action/ for more information.
To read more about the young advocates mentioned at the beginning of this article, please check the websites listed below.
Gabriel Low: https://katu.com/news/local/teen-stops-in-portland-during-cross-country-bike-ride-raising-awareness-of-rare-diseases
Taylor Kane: https://sjmagazine.net/women/girl-power-profile-taylor-kane
Harjot Randhawa: https://rarediseases.org/early-diagnosis-perspectives-from-nord-student-chapter-leader-harjot-randhawa/
Christopher Anselmo: https://www.statnews.com/2016/02/29/rare-disease-dysferlinopathy/



















Sorry, comments are closed for this post.