The first line of treatment for endogenous Cushing’s (caused by a tumor inside the body) is surgery to remove the tumor. More often than not, though, the patient’s medical journey does not end there – about a third of pituitary patients will not have a successful first surgery, around a third of initially successful surgeries will result in recurrence within ten years, and repeat surgery has a statistically lower success rate than an initial surgery.
Questions come up when high cortisol persists – is the patient a candidate for additional surgery? Radiation? Medical therapy? Options for the latter are a somewhat recent addition to the Cushing’s treatment toolbox, with the majority of research, development, and approvals happening in the last decade. Older drugs that have been “repurposed” to treat Cushing’s are also available.
Medication can be considered as a “bridge” therapy – to bring severe cortisol levels down before surgery, or in the event of a delay for any reason such as we have seen since the start of 2020 with COVID delaying appointments and surgeries, sometimes for months. It can be used to control cortisol while slow-acting radiation works (which usually takes three or more years to begin to see biochemical control). In some cases a tumor cannot be seen in scans and the surgeon might want to wait until it becomes visible to operate. There are even some patients on long-term low-dose therapy for otherwise untreatable, moderate levels of hypercortisolism. CSRF strongly encourages thorough self-education on the topic combined with conversations with your doctor when considering taking any of these medications. The choice to use a drug, or not, is a personal one. The integrity of data sources available to us varies greatly, so we cannot stress emphatically enough to always make sure your information is coming from an academic or medical source, or from a non-profit organization supported by a reputable medical advisory board.
What follows is information on each of the available medications plus several currently in development. Unfortunately, all medications are not available in all countries. Sometimes an endocrinologist might be uncomfortable prescribing one or more of these drugs because they do not have experience; all of the meds require careful dose titration and regular patient monitoring for safety and effectiveness. All of the options can lower cortisol too much and patients need appropriate instructions in the case of adrenal insufficiency.
Each drug works a little differently than the others – via mechanism of action, safety, tolerability, drug-drug interaction potential, route of administration, and how quickly it works. Most medical therapies consider a patient having a normal 24-hour urine free cortisol (UFC) test to be efficiently managed.
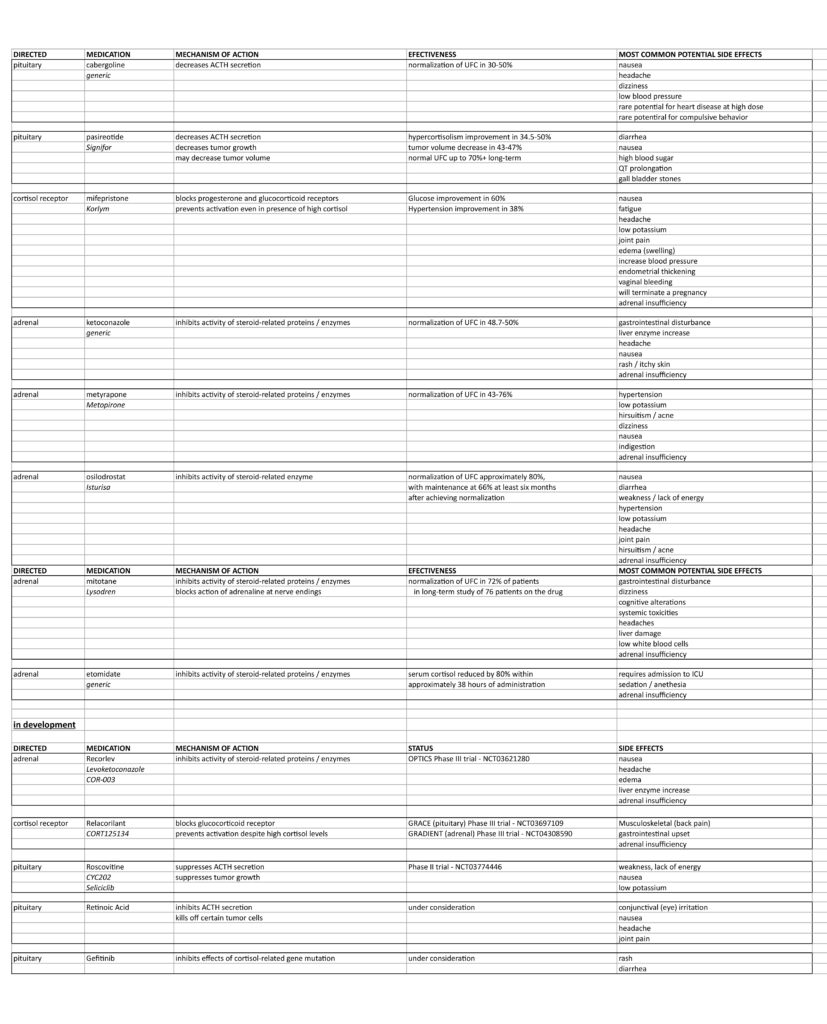
Data in the table and article taken from the recent articles “Adrenally Directed Medical Therapies for Cushing Syndrome” by Dr. Nicholas Tritos and “Updates in the Medical Treatment of Pituitary Adenomas” by Dr. Maria Fleseriu; both doctors have also reviewed this article for accurate translation. Please see references at the end of this article.
TARGET: PITUITARY
Cabergoline (generic)
-approved for management of prolactinomas, another type of pituitary tumor that produces too much of the hormone prolactin, but used “off label” for its efficacy in mild to moderate cases of Cushing’s Disease
-within 2-3 years of use, more than 75% of patients tend to become “immune” to the ACTH-decreasing effects of cabergoline
-usually used in combination with other drugs or when a patient is pregnant, since cabergoline does not appear to adversely affect the fetus based on very limited safety data available (so few patients have taken it during pregnancy, there’s no significant research on this topic)
Pasireotide (Signifor, Signifor LAR – Recordati Rare Diseases)
-injectable
-both daily and long-acting versions are FDA-approved for the treatment of Cushing’s Disease
-more than 2/3 of patients with macroadenomas (tumors 1cm or larger) saw at least a 20% reduction in tumor volume 2-3 years after beginning treatment with pasireotide
-can take up to two months to see effects, does not work for some patients
-more than 70% of patients see some sort of increase in blood glucose levels which could lead to a need for treatment to control new prediabetes or diabetes – this effect reverses upon discontinuation of use but should be monitored
TARGET: GLUCOCORTICOID RECEPTORS
Mifepristone (Korlym – Corcept Therapeutics)
-FDA approved for patients with diabetes or glucose intolerance and hypercortisolism
-studies show a majority of patients having significant improvement in glucose metabolism, blood pressure, and weight reduction / normalization
-increases ACTH and cortisol in the body, so management requires doctors to monitor clinical data and patient-reported symptoms since cortisol testing is not useful
-female patients must take extra precautions to prevent pregnancy and problems with endometrial thickening that can be caused by the antiprogestin properties in this medication
-a high dose of dexamethasone (2-10mg daily) should be used if a patient experiences adrenal insufficiency
-patients with macroadenomas (greater than 1cm) should be closely monitored for tumor growth
TARGET: ADRENAL
Ketoconazole (generic)
-common antifungal
-licensed as treatment for Cushing’s in several European countries but use is “off label” in the US
-effectiveness is supported by many studies over time including a large one that involved 200 patients followed over 17 years
-40-50% of patients see improvements in blood sugar and blood pressure
-ketoconazole requires gastric acid for absorption, so patients with existing deficiencies should take their medication with an acidic beverage to improve absorption
-many drug-drug interactions make it vital that a patient’s full medication list is discussed with their doctor
-ketoconazole can lead to low testosterone or gynecomastia in male patients
-this medication is ideally avoided during pregnancy because it may interfere with masculinization of a male fetus
-tends to increase liver enzymes, but this is usually asymptomatic and reverses upon discontinuation of the medication; about 13% of patients have mild elevation, with more severe issues (five times upper normal limit) happening less frequently
-a very rare but severe, life-threatening inflammation of the liver has been reported in 1:10,000-15,000 patients treated with ketoconazole; the FDA requires “black box” labeling because of this potential severe adverse effect, and it is unavailable in some countries because of it
-regular monitoring of liver enzymes is advisable because of the potential for serious liver injury (as noted above)
Metyrapone (Metopirone – HRA Pharma)
-rapid onset of action – begins to take effect within a couple of hours of first dose
-licensed in many European countries but “off label” use in the US, FDA approved as an ACTH test
-effectiveness is supported by many studies including one that involved 195 patients tracked over 16 years
-does not seem to have any major drug-drug interactions
-advisable to monitor blood pressure, potassium, and testosterone while on this medication
Osilodrostat (Isturisa – Recordati Rare Diseases)
-FDA-approved for use in Cushing’s in the US and also used in seven European countries for patients with active disease after surgery and those who are not candidates for surgery
-favorable effects on weight, patient-reported quality of life, blood pressure, and blood sugar have been reported
-many drug-drug interactions exist and should be discussed with your doctor
-a drop in white blood cell count (asymptomatic) has been noted in some patients
-some patients have reported minor QT prolongation, but no serious arrhythmias (both related to the healthy speed of your heart beat)
-it is advised for patients taking osilodrostat to monitor and maintain healthy electrolyte levels, monitor blood pressure, have periodic electrocardiograms, avoid other medications known to cause QT prolongation, and male patients monitor testosterone
Mitotane (Lysodren – Strongbridge Biopharma)
-derivative of insecticide, chemically related to DDT
-approved for use in adrenocortical carcinoma (cancer), rarely used outside of this condition
-usually used along with surgery to help prolong survival
-there is evidence that mitotane provides some pain relief for patients who can’t have surgery or whose tumor has metastasized
-is used in some countries “off label” for Cushing’s
-has shown some improvements in blood pressure and blood sugar
-has slow-onset effectiveness of several weeks, so not useful in urgent situations
-adrenal insufficiency occurs frequently with long-term therapy, so cortisol replacement is usually advised
-mitotane also clears hydrocortisone quickly, so a higher than normal replacement dose would be required
-many drug-drug interactions to discuss with your doctor
-it is advisable for women who may wish to become pregnant to avoid doing so within five years of discontinuing use of mitotane
-limited patient tolerance prevents widespread use in patients with non-malignant sources of hypercortisolism
Etomidate (generic)
-intravenous, used to induce anesthesia
-“off label” use for severe hypercortisolism in acutely ill patients
-only available via IV and requires admittance to ICU
-usually used as a life-saving bridge to another treatment – usually surgery – in patients with severe Cushing’s
TARGET: FUTURE APPROVAL VIA CLINICAL TRIALS
The future of more and better drug options depends on innovative researchers, adequate funding and interest in our rare disease, and patient participation in clinical trials to ensure safety and efficacy. In our Summer 2018 issue we described the design and stages of clinical trials, and it warrants a reprint here:
Trials occur in phases, or stages, based on study objectives, participants, and other factors (observational studies do not operate in phases):
- Early Phase 1: exploratory studies conducted before the traditional trials begin, involve very small doses of the drug and make no claims of therapy or diagnosis of any disease
- Phase 1: studies focused on the safety of a drug, usually conducted with healthy volunteers, main goal is to study adverse events, their frequency, and how the body breaks down and gets rid of the drug
- Phase 2: studies focused on gathering preliminary data in patients with the disease, frequently involve some patients receiving a placebo, measuring safety and adverse events still a priority
- Phase 3: studies that receive the benefit of data from the first few phases of trials and involve more participants across specific populations at different dosages, measuring safety and efficacy of the drug still a priority
- Phase 4: trials that occur after FDA approval of the drug that continue to gather safety and effectiveness data to ensure optimal use of the drug
There are several chemicals currently in various phases of clinical trial and study – see the table for more information. You can read all the data available on a trial by searching the identifier code at clinicaltrials.gov. We will feature a more in-depth look at the data behind these drugs in our next issue. A few in particular deserve a spotlight:
Recorlev (Strongbridge BioPharma)
Levoketoconazole, COR-003 – clinicaltrials.gov identifier: NCT03621280
Strongbridge BioPharma is currently running an open label, third Phase III trial (OPTICS) building on the efficacy and safety data from the previous two Phase III trials on this adrenal-targeted therapy (SONICS and LOGICS). On September 8, 2020, Strongbridge issued a press release that excellent results from the first two trials had put them on track to submit a new drug application for Recorlev to the FDA which they did in May 2021. There is an expected launch of the drug in early 2022.
Relacorilant (Corcept Therapeutics)
CORT125134 – clinicaltrials.gov identifier: NCT03697109 (GRACE – pituitary) and NCT04308590 (GRADIENT – adrenal)
Corcept Therapeutics is currently running two Phase III trials (GRACE and GRADIENT) looking at the safety and efficacy of Relacorilant, a cortisol receptor blocker that does not have the antiprogestin effect of Corcept’s original Cushing’s drug Korlym. Phase II trials for this drug completed in late 2019, and the GRACE arm is expected to complete by end of 2021. As GRADIENT began recruiting on July 28, 2020, those results will likely take a little longer. The results of the GRACE study are expected to be the basis for a new drug application to the FDA.
Roscovitine (no associated pharmaceutical company)
Seliciclib, CYC202 – clinicaltrials.gov identifier: NCT03774446
Sponsored by Cedars Sinai Hospital in Los Angeles, CA, Dr. Shlomo Melmed and his team are continuing their long-term research into the ACTH suppression and growth and size reduction effects of roscovitine on pituitary tumors in a Phase II trial. In 2011 this team reported on their creation of a transgenic zebrafish pituitary tumor model, which is remarkable because it is otherwise incredibly difficult to access enough healthy and diseased pituitary tissue to do significant studies.
References:
Nicholas A Tritos, Adrenally Directed Medical Therapies for Cushing Syndrome, The Journal of Clinical Endocrinology & Metabolism, dgaa778, https://doi.org/10.1210/clinem/dgaa778
Gheorghiu ML, Negreanu F, Fleseriu M. Updates in the Medical Treatment of Pituitary Adenomas. Horm Metab Res. 2020 Jan;52(1):8-24. doi: 10.1055/a-1066-4592. Epub 2019 Dec 20. PMID: 31863423.




Sorry, comments are closed for this post.