Introduction
Venous sampling has an important role in the diagnosis and treatment of Cushing’s disease. The purpose of the procedure is to determine the source of ACTH that causes pituitary-dependent Cushing’s syndrome, the particular form of which is given the name of Cushing’s disease. With rare exceptions, pituitary-dependent hypercortisolism is caused by an ACTH-secreting pituitary adenoma, also called a corticotroph adenoma. Diffuse overactivity of pituitary corticotrophs is recognized as a rare cause of Cushing’s disease (< 1% of cases), but for present purposes let’s assume that if a patient has Cushing’s disease, the cause is a corticotroph adenoma. To complicate matters, non-pituitary tumors, both benign and malignant, can secrete ACTH to produce Cushing’s syndrome, and this is termed ectopic ACTH secretion because ACTH is being secreted by cells that are outside of (ectopic) the confines of the pituitary gland, which is the only normal site for corticotrophs. Ectopic ACTH secretion can mimic the endocrine and clinical characteristics of true Cushing’s disease: therefore, when a high resolution MRI scan fails to disclose an adenoma, the reason can be either a corticotroph adenoma that is too small to see or an ectopic source of ACTH. Because ectopic sources of ACTH are rare in childhood, pediatric endocrinologists seldom use venous sampling in making the diagnosis of Cushing’s disease because the other causes of Cushing’s syndrome can be identified with high accuracy in the pediatric age group.
Indications For Venous Sampling
MRI scans (obtained on 1.5 Tesla scanners using thin tissue slices and with contrast enhancement) have high resolution and are capable of detecting microadenomas as small as 2.0-3.0 mm., but the tumors of Cushing’s disease are often below the scanner’s sensitivity and are missed. In our experience, somewhere in the range of 50% are missed. Furthermore, in the normal population 10-15% of individuals have a silent, asymptomatic, functionless adenoma or cyst that is visible on a high resolution scanner, which is termed a “false positive” scan. Typically, these are seen in practice as a coincidental finding when a scan is ordered to investigate the cause of headache, and most are less than 3mm in diameter. In other words, with 10-15% of scans on nomal people showing “microadenomas” or cysts, you can understand that the same number (10-15%) of patients with Cushing’s disease would have a “false positive” scan, which could lead to an operation for removal of an incidental adenoma or cyst that had nothing to do with the patient’s Cushing’s disease. Practically speaking, if a patient has a typical endocrine profile and clinical presentation, a microadenoma greater than 4mm detected on MRI scans is presumed to represent a corticotroph adenoma causing the disease. However, if either the endocrine profile or the clinical picture is atypical, the “microadenoma” may be a misleading “false positive” with the possibility of an operation that does not result in removing the cause of the disease. About 10% of the corticotroph adenomas that cause Cushing’s disease are macroadenomas measuring 10mm (1.0 cm) or more, and when the tumor is in this size range, venous sampling is seldom indicated because the incidental adenomas that lead to confusion typically are the smaller microadenomas.
The Procedure
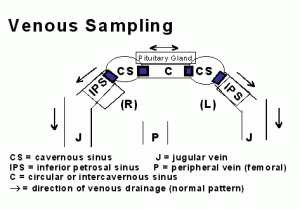
Two navigable catheters are introduced through needle punctures into right and left femoral veins at the groin. From it’s point of introduction in the femoral vein, each catheter is navigated into the jugular vein at the skull base. At the base of the skull, the catheters, one on each side, are directed into the inferior petrosal sinuses (IPS). At this point, there are two schools of thought regarding how much higher catheters should be directed to obtain the sample closest to the source of ACTH, which is the pituitary gland. As shown in the diagram, the pituitary gland occupies a position between the left and right cavernous sinuses, and blood leaving the pituitary gland enters the cavernous sinuses before passing downward into the inferior petrosal sinuses, through the jugular vein, and on into the heart.
One school maintains that sampling in the inferior petrosal sinus is adequate, whereas the other school maintains that sampling of the cavernous sinuses is more accurate. Technically, sampling the IPS is less demanding than sampling higher in the cavernous sinus, and in general angiographers prefer to stop in the IPS rather than going higher.
Neuroangiographers with greater experience can, with few exceptions, obtain samples directly from the cavernous sinuses, and do so because of a slightly higher accuracy in lateralizing tumors to one or the other side. At UCSF we are convinced that either with or without the use of CRH (corticotrophin releasing hormone), cavernous sinus sampling has a significant advantage. The IPS sampling advocates argue that while it is more accurate in a small number of cases, cavernous sinus sampling carries greater risk. In our large series of cavernous sinus sampling and the more recently reported series of Graham, et al. (Journal of Clinical Endocrinology and Metabolism 84:1602-1610) no complications occurred, but theoretically the higher site of sampling does carry greater risk, however slight. In our opinion, the theoretically greater risk is offset by its advantages.
Blood samples are taken simultaneously from a peripheral vein, typically from a femoral vein, and from the cephalic site (IPS or cavernous sinus) on both sides giving 3 samples obtained at the same time. The venous ACTH values can be compared in two ways: (1) head (cephalic) to peripheral, and (2) left cephalic to right cephalic. The first comparison is the basis for determining that the excess ACTH is being produced in the head, specifically by a tumor in the pituitary gland, and the second comparison is between the two sides. Although different ratios have been used by various investigators, in general a 2:1 before and 3:1 after CRH cephalic to peripheral ratio (or gradient) is considered proof positive that the excess ACTH is being secreted from a pituitary source. Similarly, a 2:1 side to side gradient indicates that the adenoma secreting the excess ACTH is on one or the other side of the anterior pituitary gland, which is termed lateralization. Whereas, a cephalic to peripheral gradient of 2:1 or greater provides a certain diagnosis, the 2:1 determination of lateralization is less than 100%, which is understandable given the variation in venous drainage patterns, particularly when the tumor is close to the center of the gland. With cavernous sinus sampling, correct lateralization is validated at operation in 70-85% of cases either by exposing the adenoma or by removing the appropriate one-half of the anterior pituitary gland (hemi-hypophysectomy). With inferior petrosal sinus sampling, lateralization is correct in a smaller proportion of cases, 40-70% in published reports.
Many institutions, including mine, obtain samples before and after administration of CRH. Our experience is similar to that of Graham et al.: CRH stimulates ACTH secretion by the tumor and consequently raises the differences between cephalic and peripheral ACTH values and side to side values. In a small number of patients, certainly 10% or fewer, CRH administration reveals critical differences that definitively separate ectopic from pituitary sources. CRH is expensive, but its value in even a very small proportion of cases justifies its use routinely.
Surgical Implications Of Venous Sampling
An unequivocal cephalic gradient excludes an ectopic tumor, and with confidence a transsphenoidal operation can be advised and undertaken. With no further information, the surgeon either depends on identification of the adenoma on preoperative MRI scans, or the operation becomes an exploration in which the tiny adenoma is sought by making multiple incisions into the anterior pituitary gland. However, even knowing that the adenoma is “somewhere in the anterior pituitary”, the most experienced and skilled pituitary microsurgeon may fail to find it because of its small size. Physically, the pituitary gland cannot be diced into multiple tiny pieces and retain function, and the practical limit is making incisions 2.0 mm apart as well as looking over all surfaces of the gland. Therefore, an adenoma smaller than 2.0 mm can escape detection. So what does the surgeon do in the face of a negative exploration? This is where information on lateralization is invaluable. If the ACTH gradient is 2:1 or greater, removal of the appropriate one-half of the anterior pituitary gland has a high probability of curing the patient as indicated above. Assuming that the pituitary gland is normal, which it should be in a patient with Cushing’s disease only, normal pituitary function can be maintained with approximately one-third of the gland, and this includes all pituitary functions including fertility. Without information on lateralization, in the event of a negative exploration, the neurosurgeon can either back out, in which case the patient is no better off that before the operation, or remove the entire pituitary gland, which is rarely indicated because of the adverse metabolic consequences of total panhypopituitarism.
Summary
Venous sampling, with or without CRH, has been established as a highly useful diagnostic tool in selected cases, and as we and others have gained greater experience, we are using it more rather than less frequently. It is the gold standard of diagnosis, and the additional information that is obtained by lateralizing the source of secretion is valuable in the event of a negative exploration. Even with every bit of information that it is possible to obtain, including the use of CRH stimulation, the diagnosis and treatment of Cushing’s disease remains a difficult and humbling undertaking.
Authors: Dr. Charles B. Wilson, MD, Neurosurgery; Dr. J. Blake Tyrrell, MD, Endocrinology; Dr. Christopher Dowd, MD, Neurovascular Radiology, UCSF Medical Center San Francisco, CA 94143 (Summer, 1999)




Sorry, comments are closed for this post.