Inferior Petrosal Sinus Sampling (IPSS) plays a vital role in the diagnosis and treatment of Cushing’s disease. Cushing’s disease/Cushing’s syndrome is one of the most challenging diagnosis’ in all of Endocrinology. Testing to aid in the diagnosis can be extensive, time consuming, and can take months to complete. Initially, hypercortisolemia (overproduction of cortisol in the blood) must be proven prior to any localization or cause of the symptoms to be explained. Three tests emerge as the “gold standard” in confirmation of hypercortisolemia:
- Elevation in 24 hour urine free cortisol collection,
- Lack of suppression of cortisol to <1.8mg/dL when 1 mg of dexamethasone is taken at 11pm with labs drawn at 8am
- Lack of suppression of late night (11pm-midnight) salivary cortisol signaling the loss of the normal diurnal variation in cortisol secretion.
Urine collection is cumbersome and can be affected by multiple factors including exercise, stress, kidney issues, and hydration. It can be difficult to perform given the need to collect 100% of urine produced in a 24 hour period, especially when work and other commitments intervene. Classic lab results signaling a diagnosis of Cushing’s would include urine levels >4 times the upper limit of normal, though this does not always occur and levels can be quite lower in many cases. Oral estrogen, such as that found in birth control pills or hormone replacement therapy (HRT), can also affect blood and urine results by increasing the cortisol binding globulin (CBG) which is the carrier protein for cortisol leading to falsely elevated levels of cortisol within the blood and urine. Abnormal sleep-wake cycles or variations in work shifts such as night shift workers can also lead to difficulty in performing and interpreting test results.
Once hypercortisolemia is confirmed with the above testing, and this can take many months with many discordant results/conflicting test results including some normal, abnormal, and some with minimal abnormalities, the establishment of ACTH dependence should then occur. ACTH dependence helps to confirm the cause and location of the tumor.
Cushing’s syndrome is a non-specific name for any source of excess cortisol while Cushing’s disease is specific to a pituitary source of excess. 80% of the time, Cushing’s syndrome is caused by an ACTH-dependent source with 85% of those being caused by a pituitary source and 15% by an ectopic source such as a rare lung tumor. 20% of Cushing’s syndrome is caused by an ACTH-independent source with adrenal tumors and exogenous use of glucocorticoids comprising the majority of these etiologies. ACTH levels should be drawn. Levels <5pg/mL signal an ACTH-independent cause, levels >15pg/mL signal an ACTH-dependent cause, and levels between 5-15pg/mL are in the “gray zone” where overlap can occur between ACTH-dependent and ACTH-independent causes.
Centralization or localization to the pituitary occurs next in ACTH-dependent disease. This typically occurs with one of two tests, an 8mg overnight dexamethasone suppression test similar to the previously completed 1mg overnight dexamethasone suppression test, or a CRH stimulation test. Once these tests confirm a central or pituitary source, MRI imaging of the pituitary is performed.
MRI imaging of the pituitary is recommended at facilities with special pituitary protocol that takes smaller cuts through the sella or bony region in the brain where the pituitary resides. It is recommended that facilities with Radiologists/Neuroradiologists with experience in interpretation of MRI of the pituitary be utilized. IV contrast called gadolinium can further help to identify the tumor. Pituitary tumors can be common and can occur in 10-20% of the population. Further confirmation that this tumor identified on MRI imaging is the source of the oversecretion of ACTH may therefore need to be completed and an IPSS may be ordered.
Indications for IPSS also include no obvious tumor on MRI imaging. Though this may be disconcerting to find, it is not uncommon and can occur in >50% of patients with Cushing’s disease. Additionally, small pituitary tumors <6mm may require further confirmation as the source of the hypersecretion of ACTH and IPSS can be utilized for this purpose. Size of the tumor does not necessarily correlate with ACTH overproduction. IPSS should not be used to establish the diagnosis of Cushing’s disease. 30% of the general population without Cushing’s disease can have aberrant testing on IPSS that can be interpreted as being compatible with Cushing’s disease. Therefore IPSS should be used in conjunction with the above testing to confirm a pituitary source of Cushing’s and not for the diagnosis of hypercortisolemia.
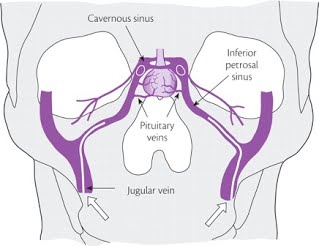
Reprinted with permission from the Imperial Centre for Endocrinology (London, England): website
IPSS is an invasive test best performed in centers with great expertise and experience performing the procedure to best decrease the chance for complications. Complications are rare though given the location of the sinus being sampled, can be significant if they occur. Though the procedure is not particularly painful, it does require catheterization of bilateral femoral veins and a peripheral vein (IV) with timing of lab draws and injection of medication, classically Corticotropin Releasing Hormone (CRH) or desmopressin. In a coordinated fashion, blood will be sampled from the right and left inferior petrosal sinus (Figure 1) as well as the peripheral vein at specified time intervals both prior and after injection. These results are then interpreted to look for an increase in the secretion of ACTH which would signal that abnormal corticotrophs (cells within the pituitary that produce ACTH) within the tumor are overproducing ACTH which then acts upon the adrenal glands to overproduce cortisol and cause the clinical signs and symptoms of Cushing’s disease. X-ray imaging is typically used to confirm appropriate localization of the catheters throughout the procedure. Additionally, blood levels can be tested for prolactin in addition to ACTH. Prolactin levels can help to confirm that the catheters are in the appropriate location. IPSS must also be performed during times of hypercortisolemia. This can be confirmed with late night salivary cortisol testing performed the night prior to the procedure and/or a 24 hour urinary free cortisol collection that begins the morning prior to the procedure and ends the morning of the procedure. The hypercortisolemia will suppress the normal corticotrophs and they will therefore not be able to respond to stimulation during the procedure. If hypercortisolemia is not present, such as can occur in the setting of cyclical Cushing’s, false positive results can occur.
ACTH levels are then interpreted in ratios. The ACTH level in the Inferior Petrosal Sinus (IPS) on the right is compared to the ACTH level in the periphery (peripheral IV). The ACTH level of the IPS on the left is also compared to the ACTH level in the periphery. Ratios >2.0 prior to the administration of CRH or >3.0 after CRH at any time point is consistent with a central or pituitary source for ACTH hypersecretion. One can then use the samples of prolactin in a similar fashion of comparing the IPS value of prolactin to the peripheral value of prolactin to confirm proper IPSS sampling. The false negative rate for IPSS is 1-10% rendering this test not foolproof but quite beneficial.
Given that the MRI can be negative or without a tumor in >50% of cases, it would be helpful to be able to lateralize or localize which side of the pituitary gland the oversecretion of ACTH hormone is coming from. IPSS is not intended to do this though. Lateralization to one side is not reliable. Venous drainage of the petrosal sinuses can have varying patterns. Lateralization rates range from 58-100%. Nevertheless, IPSS may be helpful in guiding surgical planning, especially in the setting where vague abnormalities on MRI imaging exist.
Cushing’s disease is a debilitating and complex disease. Diagnosis can be difficult, time consuming and a stressful process. Multiple tests are employed to aid in diagnosis including IPSS. Though IPSS is an invasive and expensive test, in experienced hands and the correct clinical setting, IPSS can be an invaluable tool to help confirm a pituitary source of Cushing’s even in the absence of a visible tumor on MRI. This can then lead to improvement in surgical planning and better outcomes for the patient.
Editor’s Note: Laura Knecht, MD, is the Medical Director of the Pituitary Center at Barrow Neurological Institute and maintains a private practice at Midtown Endocrine Associates in Phoenix, AZ.
Source for Figure 1: Reprinted with permission from the Imperial Centre for Endocrinology (London, England) website: http://www.imperialendo.com/for-patients/endocrine-services/pituitary-gland-disorders/the-pituitary-gland/pituitary-adenomas/cushing-s-syndrome
By Laura Knecht, MD, Summer, 2017




Sorry, comments are closed for this post.