INTRODUCTION
Androgen levels are lower in women with adrenal insufficiency than in healthy women of comparable age. This raises the question of whether testosterone or DHEA replacement therapy should be part of the standard hormone replacement therapy regimens prescribed to women with adrenal insufficiency, including in patients with a history of Cushing’s syndrome.
WHAT ARE ANDROGENS, WHAT DO THEY DO AND WHY DO WOMEN WITH ADRENAL INSUFFICIENCY HAVE LOW ANDROGEN LEVELS?
Androgens, including testosterone, are sex hormones that are responsible for secondary sex characteristics in men, such as facial hair. Although androgens are sometimes called “male hormones”, they are present in women also, though at much lower levels than in men. In fact, blood levels of testosterone are ten to 20 times lower in women than in men. In women, approximately 50% of androgens produced are of ovarian origin, with the other 50% from the adrenal glands. Therefore, women with adrenal insufficiency lack an important source of androgens and have low androgen levels. Because the pituitary secretes hormones that stimulate normal ovarian function, women with hypopituitarism lack two critical sources of androgens and typically have extremely low androgen levels. In 2001, our group showed that testosterone and free testosterone levels are significantly lower in women with hypopituitarism whether of premenopausal or postmenopausal age and whether receiving or not receiving estrogen, when compared with women of comparable age(1).
WHAT IS THE ROLE OF ANDROGENS?
Androgens play important roles in male health. In fact, men with very low androgen levels, such as those with hypopituitarism, experience many significant symptoms that are reversed by taking testosterone replacement therapy. This includes a reduction in facial and body hair, erectile dysfunction, a loss of sex drive and, in some cases, depressed mood, as well as anemia and fatigue. Low testosterone levels in men are also associated with abnormalities in body composition, specifically a loss of muscle mass and an accumulation of body fat, particularly in the abdominal (belly) area. Skeletal health is also affected, as manifested by a decrease in bone density (a thinning of the bones). However, estrogens, not androgens, are the main sex hormone in women of reproductive age, and, as noted above, androgen levels in women are only a fraction of those observed in men. This raises the question of whether androgens are important to the health of women or whether levels are simply too low to matter.
ARE ANDROGENS IMPORTANT IN WOMEN?
Relatively little is known about the role of androgens in normal female physiology or the effects of androgen deficiency (low androgen levels) in women, and no studies specifically in women with a history of Cushing’s Syndrome have been performed. However, a few studies have been conducted in women with adrenal insufficiency that may be applicable to women who are in remission from Cushing’s syndrome and have developed adrenal insufficiency and/or reproductive dysfunction (lack of menstrual periods in premenopausal women). This includes one study by our group in
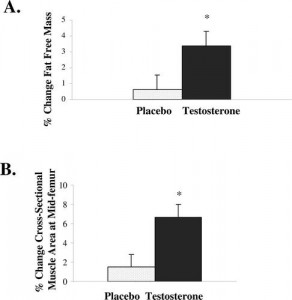
Figure 1.* Lean body mass (Panel A) and thigh muscle mass (Panel B) increased in the women who received testosterone replacement therapy compared to those who received placebo(2).
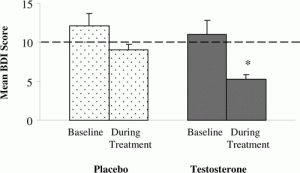
Figure 2.* Left axis: Beck Depression Index Score. Mood improved significantly in the group of women receiving testosterone replacement therapy, compared with placebo. Lower scores indicate less severe depression(2).
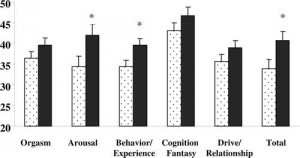
Figure 3.* Testosterone replacement therapy (black bars) was associated with an improvement in some, but not all, aspects of sexual function compared with placebo (dotted bars) (2).
In contrast, there have been about a dozen randomized, controlled studies in which women with adrenal insufficiency and/or hypopituitarism have been randomly assigned to receive DHEA, a pre-hormone that is converted to both testosterone and an estrogen by the body, or an identical placebo pill. The first of these studies was published in the premier medical journal in the country, the New England Journal of Medicine(3), and generated a lot of excitement about the potential of DHEA to improve the quality of life of women with adrenal insufficiency. In this study, 24 women with adrenal insufficiency (due to pituitary or adrenal disease) were randomly assigned to receive DHEA replacement at a dose of 50 mg per day for four months or placebo. Women who received DHEA experienced an improvement on average in their overall wellbeing, depression and anxiety compared with the placebo group(3). Likewise, DHEA improved a number of aspects of sexual function, including frequency of sexual thoughts, sexual interest and satisfaction compared with placebo(3). Subsequently, a number of additional studies were performed, some of which demonstrated positive results and others of which showed no significant improvement in quality of life in women who received DHEA compared to those who received placebo. A meta-analysis (study which combined the results of all available studies) stated that, “DHEA may improve, in a small and perhaps trivial manner, health-related quality of life and depression in women with adrenal insufficiency.” The meta-analysis also demonstrated no significant effect on anxiety or sexual function. The authors concluded that, “The evidence appears insufficient to support the routine use of DHEA in women with adrenal insufficiency”(4). This raises the question: why would some studies demonstrate a positive result and others show no effect? Consistent results would certainly be much more reassuring as to the efficacy of the medication. However, there are also other possible explanations for disparate results, including the possibility that the medication may work in some patients and not in others, and that the specific subgroup of patients who will benefit has not been identified. Other methodologic aspects of studies may also contribute to variable results, including the small sizes of the studies, doses of medications used (some studies used lower doses than the original study) and specific methods used to measure response to therapy (e.g. specific questionnaires used in the study). In the case of DHEA studies, another confounding factor is that some of the studies combined data from men and women. In the final analysis, however, one must consider the possibility DHEA may be ineffective or have an insignificant effect on quality of life in many or most women with adrenal insufficiency.
ANDROGEN REPLACEMENT THERAPY
For patients who are thinking about taking androgen replacement therapy, it is important to be cognizant of the fact that there is no FDA-approved androgen preparation for women. The testosterone patch that our group used in the study of women with hypopituitarism was never approved for use in the US by the FDA and is no longer being manufactured. The only location in which a government agency-approved transdermal (applied to the skin) testosterone preparation for woman is available is Western Australia, where a 1% cream is available. Although there are a number of testosterone gels and creams that are FDA-approved for men, these preparations deliver doses that are much too high for women, and if used over a period of time would cause significant side effects, such as excessive body and facial hair, as well as balding. Moreover, they cannot be reliably measured to deliver a dose that will safely and consistently result in blood levels of testosterone within the normal female range. Compounding pharmacies will generally accept prescriptions for testosterone creams or gels (1%) from physicians. These preparations can be applied to the thigh or lower abdomen. However, such preparations are not tightly FDA-regulated and vary from compounding pharmacy to compounding pharmacy — and sometimes from batch to batch from the same compounding pharmacy. Therefore, if prescribed, androgen blood levels must be monitored closely. DHEA tablets can be purchased at pharmacies, some grocery stores and from supplement suppliers, but studies have shown that they contain 0-150% of the labeled amount. Therefore caution and close monitoring, including of testosterone and liver function test levels, is necessary. Also, it is important to note that oral DHEA therapy results in about a 10% lowering of HDL cholesterol (good cholesterol). Of importance, although short-term safety data are reassuring with regard to the risk of development of signs of androgen excess, such as facial hair, provided hormone level remain in or close to the normal range, no long-term (beyond 24 months) safety data of these therapies is available. The recent Endocrine Society guidelines, therefore, recommend against the routine use of androgen replacement therapy in women with adrenal insufficiency or hypopituitarism, due to “the lack of adequate data supporting efficacy and/or longterm safety”(5). It further states that if testosterone is prescribed, a three- to six-month trial, with cessation of therapy for nonresponders at 6 months, is recommended, as is testosterone level assessment, before and 3 to 6 weeks after the initiation of therapy and then every 6 months thereafter, with hormone level goals in the mid-normal female range(5).
References
- Miller KK, Sesmilo G, Schiller A, Schoenfeld D, Burton S, and Klibanski A. Androgen deficiency in women with hypopituitarism. The Journal of Clinical Endocrinology and Metabolism. 2001;86(2):561-7.
- Miller KK, Biller BM, Beauregard C, Lipman JG, Jones J, Schoenfeld D, Sherman JC, Swearingen B, Loeffler J, and Klibanski A. Effects of testosterone replacement in androgen-deficient women with hypopituitarism: a randomized, double-blind, placebo-controlled study. The Journal of Clinical Endocrinology and Metabolism. 2006;91(5):1683-90.
- Arlt W, Callies F, van Vlijmen JC, Koehler I, Reincke M, Bidlingmaier M, Huebler D, Oettel M, Ernst M, Schulte HM, et al. Dehydroepiandrosterone replacement in women with adrenal insufficiency. The New England Journal of Medicine. 1999;341(14):1013-20.
- Alkatib AA, Cosma M, Elamin MB, Erickson D, Swiglo BA, Erwin PJ, and Montori VM. A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA treatment effects on quality of life in women with adrenal insufficiency. The Journal of Clinical Endocrinology and Metabolism. 2009;94(10):3676-81.
- Wierman ME, Arlt W, Basson R, Davis SR, Miller KK, Murad MH, Rosner W, and Santoro N. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology and Metabolism. 2014;99(10):3489-510.
Author: Dr. Karen Klahr Miller, MD, Spring, 2015
Editor’s Note: Dr. Miller is an endocrinologist with the Neuroendocrine Center at Massachusetts General Hospital, Boston, MA.




Sorry, comments are closed for this post.